Abstract
Chimeric antigen receptor (CAR) therapy targeting CD19 yields remarkable outcomes in patients with ALL. Three molecules (CD123, CD33 and CLEC12A) are currently targeted in clinical trials by CAR T cells for patients with AML. However, they do not feature expression profiles favorable as that of CD19.
An ideal target should be expressed in all tumor cells, at high density and in most patients. To prevent undue toxicity, the ideal target should not be expressed on any normal tissue, or at least not in vital tissues, including normal counterparts. This task requires comprehensive sources of antigen annotation, as well as analytical tools specifically designed to identify potential CAR targets.
To identify CAR targets in AML in an unbiased manner, we probed the AML surfaceome for highly abundant molecules with little to no expression in vital tissues.
We assembled a comprehensive database of 4,942 AML surface molecules by combining public protein repositories and our own cell-surface proteomics from 6 distinct AML cell lines. We computed molecule-specific AML/normal HSPC expression ratios by comparing the RNA expression levels of 26 genetically defined AML subtypes to normal BM CD34+ CD38- CD90+ CD45RA- HSCs and MPP, GMP, CMP, MEP progenitor cells, identifying 682 molecules. We combined three proteomics databases including both immunohistochemistry (HPA) and mass spectrometry (HPM and PDB) data, prioritizing antigens with membrane-associated sub-localization and expression data supported by multiple sources, thus removing 321 molecules. Finally, we selected top 24 molecules exhibiting low average expression across 43 clusters of normal tissues, and no high expression in any normal tissue, excluding blood, bone marrow and spleen.
We further defined the expression of these candidate targets in a panel of 30 primary AML samples and AML LSCs, and focused on nine candidate molecules with >75% expression in most patients. Four of these, ADGRE2, CCR1, CD70 and LILRB2, showed <5% expression in normal BM HSCs and T cells, which is critical to prevent HSC toxicity and T cell self-elimination.
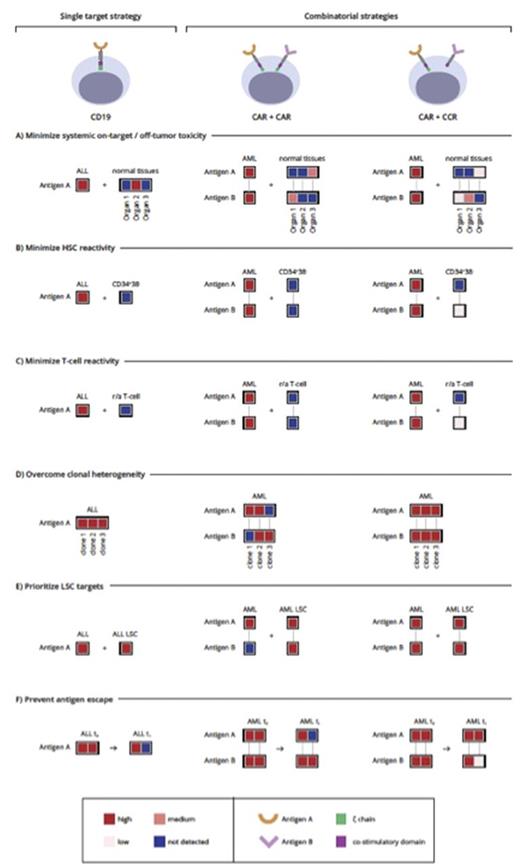
While they may have therapeutic potential, they are not ideal or as good as CD19. This prompted us to explore combinatorial targeting strategies, which fall in two major categories (Figure 1).
One is based on cumulative CAR targeting through the generation of bi-specific T cells that co-express two CARs and recognize target cells expressing any of two given antigens (CAR/CAR). Some low or moderate expression in normal tissues, albeit not optimal, may be tolerable depending on the tissues in question. The other takes advantage of split signaling to target two antigens, using one antigen to direct costimulation to enhance or rescue the suboptimal function of a CAR or TCR targeting the other antigen. In the latter approach (CAR/CCR), T cells are more restricted to dual-antigen positive tumor cells, thus relaxing the expression criteria for at least one of the paired antigens. However, pan-tumor expression of the CAR target is required. In both instances, target pairings depend on the systemic expression and co-expression of the two prospective matches to minimize cumulative expression in normal tissues.
We optimized target selection by pairing targets with non-overlapping expression in normal tissues. Starting from 12 molecules with the best expression profiles (9 candidate targets described above in addition to CD123, CD33, and CLEC12A), the 66 possible pairings, yielded few promising therapeutic combinations.
We studied four combinations: CD33+ADGRE2, CLEC12A+CCR1, CD33+CD70, and LILRB2+CLEC12A, which exhibit expression profiles unlikely to exacerbate on-target/off-tumor activity of either target alone and stained <5% of normal HSCs and T cells. Three pairings positively stained >97% of cells in AML samples, significantly above either marker alone. It is however noteworthy that total positivity was significantly higher than dual-positivity, suggesting the presence of cells expressing one antigen only. This finding is consistent with AML clonal heterogeneity and favors using such antigen pairs in the dual-targeting approach (CAR/CAR).
This present study represents a new approach to the discovery of CAR targets and will help advance the development of CAR therapy for AML and other cancers.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal